Aichuang’s coding system boosts the electronic supervision of Zhongyi Pharmaceutical’s pharmaceuticals
[ad_1]
1. Project background:
Guangzhou Zhongyi Pharmaceutical Co., Ltd. is a large-scale modern Chinese medicine enterprise with a history of more than 300 years. It is a subsidiary of Guangzhou Pharmaceutical Group Co., Ltd. and a wholly-owned subsidiary of Guangzhou Pharmaceutical Co., Ltd. One of the top 100 national pharmaceutical companies, the country’s largest research and development and industrialization base of Chinese patent medicines for diabetes, and a national high-tech enterprise.
It produces more than 130 varieties in 6 dosage forms, and has formed a product series dominated by 5 product lines for the treatment of diabetes, digestive tract, and ENT medicine, including 18 varieties of “National Basic Drug List”, “National Basic Medical Insurance, Work Injury Insurance” There are 52 varieties in the “Catalogue of Maternity Insurance Drugs”. Its flagship product, “Xiaoke Pills”, accounts for more than 78% of the entire diabetes oral Chinese patent medicine and integrated Chinese and Western medicine market sales. It is the first brand of oral hypoglycemic drugs with the most widely used population in China, with a cumulative sales volume of over 600 million bottles. There are more than 20 million patients taking it.
Zhongyi Pharmaceuticals took the lead in proposing the concept of the entire industry chain of drug quality supervision, pointing out that drug quality is a systemic project with a perpetual cycle. In this life circle, it must form a non-disruptive process from drug planting, research and development, production, circulation to the terminal. , The barrier-free industry chain system of responsibility, advocates full-variety coverage sampling and full-variety electronic supervision of essential drugs.
Wu Changhai, general manager of Guangzhou Pharmaceuticals and chairman of Guangzhou Zhongyi Pharmaceuticals, said, “Our product quality management is the concept of full-process quality management, from the cultivation and procurement of medicinal materials to the transportation and storage to the production, circulation and use of products. , We pay attention to quality management in every aspect, especially as the exclusive variety of essential medicines, “Zhongyi Quebec Pills”, we have long started to accept electronic supervision and technology.”
2. Project features:
The biggest feature of Zhongyi Pharmaceutical Co., Ltd.’s coding system production line is large output, fast packaging line speed, and many related systems. The annual data volume of Zhongyi Pharmaceutical is about 90 million, and the data volume reflected in the system exceeds 900 million. The coding system needs to process a huge amount of data, so Oracle database is used. The maximum speed of Zhongyi Pharmaceutical’s packaging line reaches 350 boxes/min. The coding system needs to scan and correlate data at this speed. At the same time, the company also uses SAP ERP, Microsoft CRM, and three-dimensional warehouses. The basic electronic supervision code assignment system of Zhongyi Pharmaceutical has also expanded the related anti-counterfeiting and anti-smuggling system.
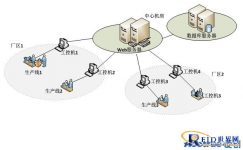
1. Cross-business system integration
Prior to the code assignment project, Guangzhou Zhongyi already had multiple business systems such as SAP ERP, CRM, etc., which were independently operated to handle related business processes.
In addition to collecting data as a production line execution system, the coding software also serves as a data exchange center at the execution level: not only must open interfaces for various business systems to provide data; but also must be able to connect to the interfaces of these business systems to import data.
2. Huge annual production
(1) Large number of single products
Exclusively produced “Xiaoke Pills”, which accounted for more than 78% of the total diabetes oral Chinese patent medicines and integrated Chinese and Western medicine market sales, is the first brand of oral hypoglycemic drugs with the most widely used population in China, with a cumulative sales volume of over 600 million Bottle, over 20 million patients have been taking it.
(2) There are many varieties
It produces more than 130 varieties of 6 major dosage forms, with an annual output of over 500 million bottles.
3. Complex production line management mode
(1) Complex and diverse workstations
Multi-station and multi-mode flexible configuration to realize the multiplexing of different products on the same production line.
(2) Strict management
Production tasks and production plans require multi-level review and approval.
(3) Fast production speed
The minimum packaging quantity is more than 350 boxes per minute.
(4) Fully automated unattended production
The entire production line adopts automatic assembly line production, which can realize unattended production.
4. “Distributed production, centralized management” management mode

5. Management of production-related enterprises
Through the Aichuang TTS management traceability platform, seamless connection with corporate warehouses, printing houses, distributors, and related companies can be achieved.

[ad_2]



