Drug Supervision Code of Jiangsu Provincial Department of Health
[ad_1]
Case background:
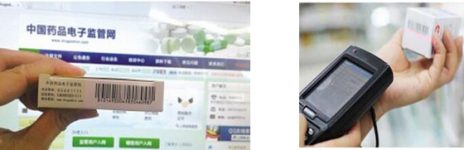
The electronic drug supervision code refers to the electronic drug supervision code that the manufacturer must print (stick) a uniform mark on the smallest sales package of the listed product. It is a barcode with 20 numbers starting with 8 and is generally printed on both sides of the outer packaging of the drug . Different from the previous barcode, even for the same brand of medicine, each box of medicine has a different code number, which becomes the only “identity card” for medicines. Pharmaceutical wholesale companies and retail companies must scan this supervision code when purchasing and shipping goods, and collect and report data through the unified national supervision network. In this way, the entire process of each box of medicines, from production and delivery, circulation, transportation, storage to distribution to medical institutions, is under the supervision of the drug regulatory authority. Once a problem drug is found, it can be quickly found and recalled to realize drug traceability and ensure drug safety.
Case introduction:
The electronic drug (vaccine) supervision system is a national platform for people’s livelihood, and it is a complex system involving many government units, drug business operators and consumers. The implementation of the electronic drug supervision code has gone through 4 stages. The first stage is the construction of the system platform, the second stage is the drug production data management of the drug manufacturer (the drugs in the catalog), and the third stage is the drug information management of the hospital medication. The fourth stage is the distribution channels and logistics and retail management of pharmacies. The company’s barcode scanners and wireless barcode handheld terminals have achieved rapid automatic data collection of electronic drug regulatory codes for the majority of drug business units, hospitals, pharmacies, etc. in the second, third, and fourth stages, and are in line with the national drug regulatory platform. Successfully seamlessly connected, it has been safely and reliably applied in many hospitals and health centers under the jurisdiction of Jiangsu Provincial Department of Health, Henan Provincial Department of Health, and some health bureaus in Gansu; it has won unanimous praise from customers in many chain pharmacies.

User benefits:
After the implementation of electronic supervision, the dynamic information of pharmaceutical production and circulation will be collected in the database in real time. The customer service center provides information technology support for the government to implement quality supervision and establishment of electronic files from the source, track and trace the market, request for evidence and invoices, implement purchase inspection and acceptance, establish purchase and sales electronic ledger, and recall problem drugs.
[ad_2]



