The application of EVOC EPX products in the Drug Administration Code Assigning System
[ad_1]
The Internet of Things refers to the ubiquitous terminal equipment and facilities, including sensors with “intrinsic intelligence”, mobile terminals, industrial systems, building control systems, home intelligent facilities, video surveillance systems, etc., and “external enabling” , Such as various assets affixed with RFID, individuals and vehicles carrying wireless terminals, etc., through various wireless and wired long-distance and short-distance communication networks to achieve interconnection, application integration, and cloud computing-based SaaS operations In the intranet, private network, and Internet environments, appropriate information security assurance mechanisms are adopted to provide safe, controllable and even personalized real-time online monitoring, positioning and tracing, alarm linkage, dispatching and commanding, plan management, remote control, and security Management and service functions such as prevention, remote maintenance, online upgrades, statistical reports, decision support, and leadership desktops realize the integration of “management, control, and operation” of “high efficiency, energy saving, safety, and environmental protection” of “everything”.

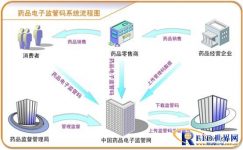
The electronic drug supervision code is a specific application of the concept of the Internet of Things. The electronic label assigns a unique electronic supervision code to each drug in the smallest package to realize “one piece, one code” management. The dynamic information of the production, circulation, and use of the drug corresponding to the supervision code is collected into the database in real time. Sewing network, ultra-large database supporting tens of billions of products of millions of enterprises, and professional customer service center, establishing electronic files for the government to realize quality supervision from the source, tracking and tracing the market, requesting certificates, and implementing Incoming inspection and acceptance, the establishment of an electronic account for purchases and sales, and the recall of problem drugs provide information technology support. Finally, an electronic custody chain for the whole process of medicines from entering the factory, production and processing, ex-factory sales to after-sales service was established. The safe traceability and accountability system provides an information technology platform and establishes an electronic drug quality supervision network covering the entire society.
The drug regulatory code data collection system can print the label on the spot during the primary packaging, or print the label in advance. Scanning the drug regulatory code of the primary packaging during the secondary packaging, the system automatically counts according to the pre-set packaging specifications, when the quantity reaches the packaging specifications, the system drives the barcode printer to print the secondary packaging code, and performs packaging and bar codes. If there is a next-level packaging in the packaging specification, the same packaging procedure is entered for higher packaging operations. If the highest level of packaging specified in the packaging specification is reached, the packaging is prompted to complete the offline. In the packaging process of each layer, the upper packaging supervision code is automatically associated with the corresponding scanned lower supervision code. The packaging supervision codes of each layer of each largest package are linked layer by layer to achieve the overall association, which can be passed through any packaged package in the system The supervisory code queries its corresponding upper-level code or lower-level code.
The drug regulatory code hardware system is composed of a server, a printing PC, a flat labeling machine, an on-site industrial computer, a drug regulatory code scanner, a barcode printer, etc. In addition, existing equipment needs to be modified according to the implementation of the system.
Based on the characteristics of the industry, the following requirements are put forward for the drug regulatory code hardware system:
1. Diversified hardware peripherals: The drug administration code assignment system consists of a variety of hardware devices, mainly including handheld, fixed, laser, barcode, label and other code/scan code devices that need to be connected to the code assignment industrial computer ;
2. High stability and reliability: The use site of the system is the production line of a pharmaceutical factory, and ordinary operators do not have professional computer maintenance capabilities. At the same time, the working status of the coded industrial computer directly affects the production process of the production line.
System Block Diagram

System Block Diagram
The specifications of EVOC EPX series product EIC-2403 are as follows:
Dimensions: 177mm(H)×482mm(W)×452mm(D)
CPU: Core2 Duo 2.4G/2.5G/2.6G/2.8G/3.0G (optional)
Memory: 2G/4G (optional)
Hard disk: 500G/1T (optional)
Optical drive: 18xDVD (optional)
Standard I/O: 2 RS-232 serial ports, 8 (16 optional) RS-232/422/485 serial ports, 4 USB, PS/2
Digital I/O: (optional)
Floor: Compatible with 13-slot, 14-slot floor
Front panel: with LCD screen, real-time temperature monitoring and operation status display of power supply hard disk
Power supply: support PS/2 power supply
Others: Two shock-proof pressure strips are used to prevent vibration
Structure: safety door with lock protection control components, keyboard interface at the front and rear of the whole machine
This product, which is professionally applied to the drug regulatory code-assigning system, has several advantages:
1. EVOC’s unique EPI bus and its interface, using European-style connectors instead of golden finger connection, 360-degree contact, sealed and dust-proof, strong structure, anti-vibration, can withstand a certain degree of gravity, to ensure a stable and firm connection;
2. Excellent air intake heat dissipation design, real-time monitoring of the temperature of the whole machine;
3. Abundant extensions, fully meet the control of the stable and convenient access of a variety of hand-held, fixed, laser, barcode, label and other code assignment/scan code equipment at the control site;
4. It supports a truly high-performance dual gigabit network, and provides a good network environment for code application/release.
In short, selecting EVOC’s uniquely developed EPI bus products for high-reliability sites can comprehensively improve the problems of gold finger oxidation, deformation, and dust caused by long-term use of traditional industrial control products. The European-style connector is used to replace the golden finger connection, 360-degree contact, sealed and dust-proof, solid structure, anti-vibration, can withstand a certain amount of gravity, and ensure a stable and firm connection. At the same time, this series of products has rich expansion capabilities. The whole machine supports PCI2.3 specification and is compatible with customers’ original functional boards, which realizes the stable improvement of overall system safety performance and stability.
[ad_2]


