Uboxun and Zhongxin Platform to help drug traceability and drug circulation supervision
[ad_1]
Project Background
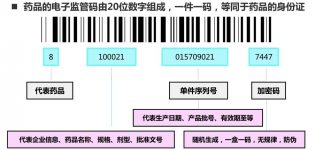
China’s electronic drug regulatory system is a third-party application platform for drug regulatory agencies, health administrative agencies, industrial and commercial administrations and other departments to supervise the entire process of drug production, circulation, and use. The above-mentioned regulatory agencies can use smart handheld terminals and other equipment To carry out data collection and management, complete the one-to-one correspondence between drugs and supervision codes, and realize drug flow traceability and emergency recalls.

Since its official operation in October 2007, China’s pharmaceutical electronic supervision system has implemented electronic supervision of pharmaceutical manufacturers, wholesale companies, and third-party logistics companies through the CITIC network access platform. However, it lacks supervision of end-use links. The Ministry of Health requires all Medical and health institutions have completed the work of joining electronic drug supervision.
Customer goals
Customers need to monitor the status of drugs in the production and circulation process to realize the traceability and management of drugs by regulatory agencies and manufacturers, so as to protect the legitimate rights and interests of drug manufacturers and consumers.

Program implementation
1. Demand analysis
This set of solutions for the drug supervision management system uses the Uboxun mobile handheld terminal PDA as the hardware core, and is equipped with strict and complete electronic supervision system software. Through the connection of the handheld terminal to the mobile network, it can query each box and each box in real time. The production, inventory, sales and logistics distribution of one box and each batch of drugs can be traced to the traced drugs throughout the process to minimize the cost of recall; and the electronic supervision system will strictly monitor the production, circulation, transportation, and delivery of drugs. The whole process from storage to drug consumption realizes the unification of drug supervision, logistics application, merchant settlement and consumer inquiry functions.

2. Process description diagram

3. Functional application
Real-time monitoring of the entire process from production and delivery, circulation, transportation, storage, and distribution to medical institutions; real-time query of the production, operation, inventory and flow of each box, box, and batch of key drugs.

4. System topology diagram
Taking the China Pharmaceutical Electronic Supervision System platform as the information center, through the data communication of the Uboxun mobile handheld terminal, the information interaction between the government, operation and clients is realized. The following figure shows the overall structure:

5. Detailed function
(1) Write-off and storage: carry out storage operations on the drug regulatory code. The program version is different, and the specific storage types are different. Common storage operations include purchase storage, return storage, and so on.

(2) Write-off out of the warehouse (sampling inspection, return): For the drug information that has been put into the warehouse, the outbound operation is required. The program version is different, and the specific outbound type is different. The common outbound operations include random inspection out of the warehouse and return. Delivery, supply delivery, retail delivery, destruction delivery, etc.

(3) Cancellation of warehouses (supply, retail, destruction)

(4) Data management (download): download basic data from the CITIC platform or from the server, such as units, medicines, data dictionaries, etc.;

(5) Data management (upload): upload the scanned drug business data to the CITIC platform or the drug regulatory code system through wireless or wired means;

Program effectiveness

Applicable Products

Industrial grade mobile handheld terminal I6100 series
[ad_2]



