Yangtze River Pharmaceutical Group adopts electronic supervision code assignment system
[ad_1]
【company profile】
Yangzijiang Pharmaceutical Group was founded in 1971. It is a large national pharmaceutical enterprise group that cross-regions, integrates production, education and research, and integrates science, industry and trade. It is also the first batch of innovative enterprises in the country named by the Ministry of Science and Technology. The group is headquartered in Taizhou City, Jiangsu Province, with more than 9,000 employees and a total area of more than 2 million square meters. More than 20 member companies are distributed in Taizhou, Beijing, Shanghai, Nanjing, Guangzhou, Chengdu, Suzhou, Changzhou and other places; the marketing network covers all provinces, cities, and autonomous regions across the country.
Over the years, the group has always been adhering to the concept of “seeking for progress, protecting all beings”, practicing the development tenet of “high-quality benefiting the people, innovation for the people”, and committed to providing the society with high-quality and efficient medicines and health services. Since 1996, the comprehensive economic efficiency of the company has been ranked in the forefront of Jiangsu Province and the national pharmaceutical industry for more than ten consecutive years, and has been among the “Top 500 Chinese Enterprises”, “Top 500 Chinese Private Enterprises”, and “Top 500 Taxpayers in China”. According to data released by the Ministry of Industry and Information Technology of the People’s Republic of China, in 2009, 2010, and 2011, Yangtze River Pharmaceutical Group Co., Ltd.’s main business income ranked among the top three in the top 100 national pharmaceutical industry enterprises for three consecutive years.
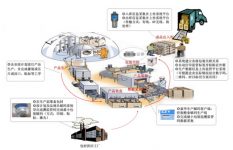
[Scheme Overview]
The electronic supervisory code coding system solution is a software-centric integrated solution that combines automatic identification technology, automatic control systems and equipment. Its content involves multiple disciplines and requires a team with comprehensive knowledge and capabilities to ensure the system’s performance. Successfully implemented.
Due to the particularity of the electronic supervision code, the solution will directly affect the production and logistics efficiency of the enterprise, whether it is from the layout of the production site, the adaptability of the business process, the flexibility and stability of the software system, the handling of software exceptions, and the flexible expansion of the software In other aspects, it will directly affect the entire production and operation process of the enterprise.
K. Wah has many years of experience in the implementation of enterprise supply chain logistics management and drug electronic supervision. According to the characteristics of Haini Pharmaceuticals, after we conducted preliminary investigations on the relevant business processes of the enterprise, we proposed this solution in a targeted manner to help the enterprise Complete the packaging and coding business process of the production line with the least investment and the highest efficiency.
【Business Framework】

K. Wah’s electronic supervision coding solution tailored specifically for the pharmaceutical industry is fully applicable to the business process of the pharmaceutical industry. The main business framework covers the following:
Supervision code application: The enterprise applies to the China Drug Electronic Supervision Network for the corresponding drug electronic supervision code;
Production code client: from the drug sub-packaging stage, the minimum packaging, transition packaging and maximum packaging are respectively assigned electronic supervision codes, and the corresponding relationship is created, and the packaging relationship data is exported at the end of production;
Inbound and outbound verification, verification and write-off: Coded products can realize the verification and verification and verification of inbound and outbound products by scanning the supervision codes of all levels of packaging;
Supervision code upload: The production code data and the warehouse verification and write-off data are uploaded through the drug supervision network client, and the electronic drug supervision process of the manufacturer is completed.
[ad_2]



