Beiyang Group RFID medical anti-counterfeiting system solution
[ad_1]
1. System background
Medicine is a relatively special product. Except for some medicines focusing on health care and nutrition, the main role of medicine is to treat diseases and save people. The problem of “difficult and expensive medical treatment” has been raised more than once in various conferences and meetings in recent years, and the drug reform among them has attracted much attention.
The false high of drug prices is the focus of everyone’s attention. In the largest forced consumer market, drugs, under the guidance of huge profits, fakes in drugs are not only natural enemies of drug manufacturers, but also make consumers frightened when buying drugs. What anti-counterfeiting methods are used and how to stop the market? The fakes on the Internet are a major proposition that the pharmaceutical industry and even the entire pharmaceutical industry are constantly discussing.
2. System overview
The pharmaceutical RFID supply chain information platform built with RFID technology can realize real-time tracking and supervision of pharmaceuticals in the production, circulation, distribution, and retail links. Because RFID electronic tags have the technical characteristics of large storage capacity, fast transmission speed, non-counterfeiting, and concurrent identification, so through the support of the supply chain platform database, and attaching RFID electronic tags to a single pharmaceutical product, it can be reliably and real-time in the tag Record drug-related supply chain information in order to conduct real-time medical supply chain supervision. The specific key data of the supply chain can be traced back: from the time the drug leaves the factory, the subsequent supply chain process, and finally reach the patient through the retailer or the hospital.
Relevant regulatory authorities can achieve the purpose of market regulation and supervision through data verification and statistics; doctors and patients can also ensure drug safety by reading the data information stored in the label; and pharmaceutical companies are implementing drug anti-counterfeiting and improving operational efficiency. At the same time, the sharing of data and information such as warehousing and circulation in the supply chain can also be used to improve work efficiency and collaboration capabilities.
The use of RFID medical anti-counterfeiting technology can prevent counterfeit products from flowing into the sales market, control product quality, supervise and manage sales personnel, formulate reasonable service strategies, strengthen market control and management, guide enterprise product design and positioning, and improve the timeliness of business decision-making.
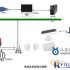
Three, the system process
The use of RFID technology will optimize the entire production, circulation, and sales of pharmaceutical products, and can obtain accurate information flow in real time, improve the monitoring during the circulation process, and effectively realize anti-counterfeiting and curb the flow of counterfeit drugs into the market.
1) Pharmaceutical companies, as drug manufacturers, are responsible for attaching RFID electronic tags to drug packaging. There are two types of RFID electronic tags for medicines, one is the HF label affixed to the small drug packaging box of each sales unit, and the other is the UHF label affixed to the packaging carton.
2) Circulation-related units (including pharmaceutical wholesale companies, pharmaceutical retail companies, and medical institutions) are equipped with special RFID identifiable reading and writing devices. Pharmaceutical business enterprises perform product authenticity identification and circulation information writing operations during product entry and exit.
3) The drug regulatory department can use hand-held (portable) identifiable read-write devices to conduct on-site inspections and identifications of drugs in the warehouse and on sale of relevant drug circulation units.
4) Manage the identity registration and issuance of digital certificates for drug manufacturers, drug distribution companies, drug retail companies, and medical institutions. The machines and tools used by various enterprises and institutions are also certified and managed through appropriate mechanisms.
5) When consumers buy medicines in hospitals or pharmacies, they can authenticate the authenticity of the medicines through the identifiable read-write machine or self-service identification terminal of the hospital or pharmacy.
6) The application software of the platform is connected with the information systems of pharmaceutical manufacturers, wholesale companies, retail companies, medical institutions, etc. through data interfaces to realize data exchange. Complete drug circulation records have been collected on the platform to realize circulation supervision and Internet authenticity inquiries.
4. Anti-counterfeiting query and sales management
1) Distributors and retailers are equipped with handheld devices to check the authenticity of medicines
The sales of medicines are the most important part of the anti-counterfeiting link. Most counterfeit medicines enter the circulation of medicines through loopholes in the sales link. It can be stipulated that medical distributors must be equipped with handheld devices, and the medicines must be inspected to ensure that no fake medicines are mixed in; at the same time, the sales of high-end medicines are often sold through large shopping malls, supermarkets, specialty stores, etc., and retailers can be recommended to also equip them (It can be rented) The hand-held machine is used to check the authenticity of the medicine. As consumers, when purchasing medicines, they have the right to require retailers to use handheld phones to check the authenticity of medicines on the spot. If the data can be successfully read to indicate that the medicine is genuine, consumers can refuse to buy if the data in the label cannot be read.
2) Establishment of inquiry agencies in medicine
The electronic label data processed by the reader will be recorded in the server, and the data processing system will be linked to the Web query system. The distributor, retailer or medical purchaser can query the ID number of the electronic label through the communication network to identify the authenticity of the drug .
Five, system characteristics
1) Tracking the medical supply process
The system can record and track the operation of pharmaceutical products in real time, accurately and completely, and can comprehensively and efficiently strengthen the management from product production, transportation, to sales and other links, and provide a variety of comprehensive and easy-to-use queries, statistics, data analysis, etc. Features.
2) Effective anti-counterfeiting function
In the sales process of medicine, since the package cannot be opened on the spot to check its authenticity, this provides opportunities for counterfeiters. However, you can clearly understand the factory location, year, date of manufacture, ingredients and other information of the drug through the information in the pasted electronic label, and realize the anti-counterfeiting identification function of the drug.
3) Storage management of medicine
Paste an electronic label on each bottle of medicine, record the placement location, product category, date and other data. According to the unique code of each product, you can keep track of the status of the goods and the quality of the medicine at any time, so as to facilitate storage management and understand immediately Items that require replenishment are convenient for out-of-stock management. And in the case of return and exchange, as long as it is poured into the system, the data can be modified.
6. Social benefits
1) Can effectively improve the level of supervision
Due to the lack of basic information, many current supervision work in the field of drug circulation can only stay at the level of post-event supervision. Using RFID technology, it is possible to achieve the organic unification of pre-prevention, in-process supervision, and post-processing, and achieve full coverage of supervision work.
2) The manufacture and sale of fakes can be prevented to the greatest extent, and the accident handling capacity can be improved
After adopting the electronic label technology, since the information in the label is difficult to be tampered with, it is difficult for these counterfeit medicines to flow into the circulation link, and it is difficult for them to enter the hands of patients. After the counterfeit medicine is discovered, it is much easier to recover and dispose of it.
3) It can effectively promote the improvement of industrial energy level
If electronic label technology is promoted in pharmaceutical companies, these companies will definitely improve their own informatization and management capabilities, which will undoubtedly be of great help to enhance the energy level of the industry and enhance the competitiveness of enterprises.
Sales Hotline: 0631-5698111
[ad_2]