GMP/GSP commodity logistics digital management solution in the pharmaceutical field
[ad_1]
I. Overview
The “GMP/GSP Digital Commodity Logistics Management Information System in the Pharmaceutical Field” adopts the coding method of the “Pharmaceutical Coding Guidelines” compiled by the China Article Coding Center, trying to establish a set of standardized work models for the commodity logistics management information system of pharmaceutical enterprises, which is comprehensive for the pharmaceutical industry. Promote the bar code standard system (EAN·UCC system), improve the logistics management level of the entire industry, play the effect of applying the exemplary system, and drive the entire industry to use standard codes and bar codes in all links of the supply chain and logistics management, so as to use systematic The method thoroughly solves the problems that have plagued the pharmaceutical industry for many years, such as counterfeit drugs, cross-stocking, capital return, imperfect management, etc., and comprehensively improves the operational efficiency and economic benefits of the pharmaceutical industry.
2. Raising the problem
The pharmaceutical industry is a sunrise industry that is related to the health of the people, develops rapidly, and has a relatively backward technical management level. According to statistics from relevant departments, medical and healthcare products account for only 6.6% of the current 80,000 barcode system members. The use rate of barcodes for medical and healthcare products is also quite low. According to estimates by experts from the China Association for Automatic Technology Identification and Prevention, one-dimensional barcodes for medical and healthcare products are used The rate is less than 50%, and the products produced by many pharmaceutical companies do not use barcodes or use self-encoding at all. As a result, pharmaceutical companies encounter great obstacles when they use logistics and commercial management efficiency and information methods in their production and operation activities. A series of management problems are derived from this, which urgently need to be solved by adopting advanced technical means and supporting administrative measures. The specific problems are as follows:
1. The problem of counterfeit and inferior medicines has always been a major problem that plagues China’s pharmaceutical consumption fields and manufacturing enterprises.
Although the country has been cracking down on counterfeit and inferior drugs, driven by interests and local protectionism, this problem has not been fundamentally resolved. Tongrentang spends tens of millions of yuan in anti-counterfeiting expenses every year. The country’s annual economic loss is as much as 100 million yuan. So far, no corresponding measures have been found to fundamentally solve the problem.
2. The occurrence of stray goods in the internal management of enterprises, the tracking of goods and the handling of returns are also problems that have long existed in the management of production enterprises and lack efficient corresponding solutions.
Pharmaceutical companies and pharmaceutical sales companies pay a lot of market risk and management costs for internal diversification. The tracking and return processing of merchandise and goods sales and services require a lot of manpower and time.-The search for diverted goods or problem products is currently In most companies, it usually takes more than one week. At present, there is no good way to prevent in advance in the place of production or to detect the occurrence of smashing incidents in advance in the place of consumption.
3. Problems of efficiency, validity management, and automatic distribution in circulation.
Chain operation and the establishment of a sales network are the most representative business operation models in modern circulation under the conditions of a market economy, and are objective requirements for reducing costs, improving efficiency, and carrying out large-scale social production. With the reform of the medical system, this kind of network chain operation is becoming a new popular way of selling pharmaceutical products. The special attributes of drugs objectively require the management of the single products and expiration dates, approval batch numbers, regions and other information implemented in the circulation, and operations such as GMP/GSP certification processing in multi-variety mixed shipment operations and receiving and dispatching operations. This makes the market-oriented circulation of pharmaceutical commodities more complicated and professional than the commodity circulation of ordinary supermarkets.
4. In the sales management of the production enterprise, the problems of the inventory, the return of funds (control of the timing of settlement), and the dynamic feedback of the production management.
In the enterprise production management, the dynamic feedback of production organization, inventory, sales progress and the grasp of the capital return status are the most concerned issues of the normal operation of the enterprise and the business operators, and how to make the dynamic information of production be accurately and timely fed back to the managers The hand is the core of the normal operation of the enterprise.
5. The cost of logistics in a general sense.
Systematization of logistics is the basis of rationalization of logistics, and rationalization of logistics is the overall goal pursued by logistics management of the entire industry chain. Logistics rationalization can reduce logistics costs and reduce the cost of merchandise sales; secondly, logistics rationalization can compress inventory and reduce the occupation of working capital; more importantly, by improving logistics, the management level of the enterprise can be improved, and the commodity can be realized in the field of circulation. It is the goal pursued by modern enterprises to provide value-added services to downstream enterprises.
6. To ensure the actual effect of GMP/GSP implementation, it is necessary to establish GMP/GSP information standards.
At present, the coding of ordinary commodities cannot meet the needs of the circulation of pharmaceutical commodities. However, some pharmaceutical companies do their own work and perform their own coding. A large number of non-professional, informal, and non-standard self-made barcodes are circulated in the market. This is a waste of resources and different companies. Can not read each other, and lose the value of the bar code itself. In the pharmaceutical chain business environment, how to prevent counterfeit and inferior drugs from entering the market through formal channels to ensure the actual effect of GMP/GSP implementation requires the establishment of GMP/GSP information standards.
7. Back-end software and data collection methods, data source issues (data authenticity issues), and incorrect and inaccurate data in reality often cause decision-making errors.
The establishment of the logistics distribution system and fast data collection methods and data sources such as storage, storage, delivery, sales, and inventory are objective factors that restrict the efficiency of pharmaceutical logistics management and business activities. At present, many companies use computers for financial and commodity management, and some companies have introduced ERP technology. However, whether business leaders using these software can make decisions correctly depends on accurate, real-time data and the source of the data. , Collection and statistical processing methods. The error rate of manual keyboard entry is 3-5% and the speed is slow, and the delayed information feedback makes the data in the management database invalid.
8. The loopholes caused by human factors in the manual GMP/GSP operation process make it a new issue to prevent counterfeit drugs from flowing into the hands of consumers through legitimate channels.
GMP/GSP procedures and operating specifications are the basis for pharmaceutical companies to ensure product quality and prevent counterfeit drugs or drugs that do not meet GMP/GSP certification requirements from entering normal circulation channels. However, at present, it mainly relies on manual input of purchase, delivery and certificate verification operations. It is not guaranteed that due to profit-driven or human errors in all aspects of the business process, drugs that do not meet the requirements of GMP/GSP certification will be allowed to flow into consumption through legitimate channels. In the hands of the operator, it is a new requirement for the system to be able to restrict and restrict the illegal behavior or wrong operation of the operator in advance. Therefore, it is necessary to find technical means to ensure through systematic methods.
Three, program introduction
Establishing a complete GMP/GSP digital management system based on bar code technology through information technology is the foundation and the only way to fundamentally solve various problems in the current pharmaceutical industry.
1. Current conditions in the industry
The GMP/GSP standard is promulgated, and the certification will be implemented before the end of 2004; the trial version of “Pharmaceutical Commodity Code” is released. With the development of medical information construction, logistics, information flow, and business flow (funds) are separated, providing us with three layers of anti-counterfeiting protection The establishment of the system provides the possibility.
2. The goal of system design
Realize the digital management of GMP/GSP in the pharmaceutical industry, and establish a “digital firewall” to prevent counterfeit drugs from flowing in through normal channels, so as to improve the overall logistics management level and credibility of pharmaceutical companies. Pharmaceutical companies take the social responsibility of preventing counterfeit and shoddy products for consumers, and use technical means to identify counterfeit and shoddy products, rather than consumers themselves. A three-layer protection system based on logistics, information flow and business flow has been established.
Solve the standardization and standardization of information and bar codes in the pharmaceutical industry, so that different versions of medical management software can be used on the same platform, so as to realize resource sharing between different systems and connection and information exchange between different systems. Realize networked data transmission and networked operations that do not require data lines.
Strictly follow the requirements of GMP/GSP to establish an efficient and standardized operation system. Including: procedures, operations, documents, etc., to establish information standards for GSP and GMP.
According to the operating characteristics of the pharmaceutical industry, we will make full use of the latest technology to construct and optimize the logistics distribution system. Realize bar code single product management and solve the problem of information system data source.
Convenient, timely and accurate collection, transmission, statistics, analysis, and reporting of logistics information during the entire operation of each link in the supply chain. On the basis of standardized, efficient and accurate data collection and analysis, it provides a reliable data basis for decision-making support to comprehensively evaluate the situation of people, finances and materials in the process of import, storage, transportation and sales. . Implement guidance and control on the operation of various links in various processes, improve efficiency, reduce costs, reduce errors, reduce labor intensity, and ensure the continuous improvement of corporate management and operational efficiency.
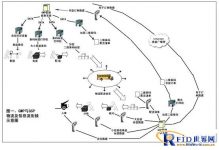
3. System composition

Schematic diagram of the application scheme—–the logistics and information flow connection between GMP and GSP
Fourth, the definition and composition of the system boundary
1. Defining the edge of the system
From the delivery management of pharmaceutical production (production field), through the entire process of the circulation basin (wholesale, retail, storage and transportation), to the receiving management of end users (pharmacies, hospitals).
2. The composition of the system
The system consists of three parts: production area, circulation area and end user.
1) Production area
◆Barcode generation system
Mark a one-dimensional bar code on the single-product medicine box, and the bar code includes text information such as bar code information, production time, batch number, and serial number;
Use two-dimensional barcodes to generate shipping and recycling orders;
Chuan cargo alarm and feedback system.
◆Barcode circulation management application system
Goods storage management: checking and reading.
◆Shipping management: two-dimensional bar code invoice generation and shipping check
◆Cargo tracking and quality monitoring: solve the problem of collocation, return, and quality tracking
2) Circulation field
◆Effectively support the new management mechanism and business process system of the super-large-scale chain system, improve efficiency, and quickly respond to market changes. Based on the dynamic website to establish the system and the supply chain B2B flat marketing model;
◆Effectively assist branch stores to carry out local purchase, sales, and inventory management, strengthen store management; effectively assist wholesale and major customer sales business;
◆Real-time grasp and monitor the sales situation of the entire system;
◆Real-time monitoring of the inventory distribution of the entire system (including strategic library inventory, distribution inventory, store inventory, etc.), effectively reducing inventory costs and controlling potential business risks;
◆Effectively support inventory management, centralized procurement and unified distribution business;
◆Integration of logistics, capital flow and information flow, support the promotion of finance from financial accounting to financial analysis, control and management, and support centralized financial management;
◆Effectively support the management of single products and strengthen the management of drugs;
◆Auxiliary performance monitoring, providing multi-dimensional analysis to assist decision-making.
3) End user
Goods storage management: checking and reading.

Schematic diagram of application scheme-sample of medical receipt list
Five, system characteristics
1. One-dimensional barcode and two-dimensional barcode are used in combination;
2. Completely integrate the business process of pharmaceutical companies, design according to GMP/GSP specifications, and comply with the basic requirements of GMP/GSP;
3. Domestically, it is the first to put forward the concept of GMP/GSP digital management;
4. The establishment of the concept of a system firewall to prevent counterfeit drugs and the use of means, the introduction of the concepts of “information protection” and “system arson wall” and the formation of corresponding plans, which fundamentally change the tracing of consumer problems and the inability to prevent them in advance Working mode. The responsibility for anti-counterfeiting is borne by the enterprise instead of being identified by the individual in the past;
5. Really realize the single product management of medicines, and the barcode single crystal management solves the data source problem of the information system;
6. In the environment of the existing enterprise management system, minimize the cost burden of the enterprise;
7. The means to prevent counterfeit medicines from entering the circulation field is to prevent the counterfeit medicines from entering the circulation and not after they reach the consumers and cause problems;
8. As a unified bar code standard, realize industry standardization and standardization, and use the standard self-executing system method to enable different versions of medical management software to be used in the same execution system learning platform to realize resource sharing;
9. Fundamentally solve the problem of occurrence and fast-tracking of drug cross-shipping;
10. Realize regionalized data transmission and networked operations that do not require data lines;
11. The establishment of a reversible commodity logistics and quality tracking and tracing management system.
6. Social and economic benefits
There are currently 6,000 pharmaceutical companies, 150,000 drug distribution companies, and 3,000 medical institutions in China. If the logistics cost is calculated as 2% of the annual sales revenue of drugs accounting for 3.5%, the management cost is calculated. The cost savings for these two items alone is about 15%, and the direct economic benefits generated by the entire industry amount to nearly 5 billion yuan throughout the year.
The implementation of this plan will benefit enterprises, consumers, and the country. Make it easier for the pharmaceutical authorities to monitor and prevent the circulation of counterfeit and inferior drugs; improve the efficiency of the company, reduce costs, simple operation, and increase revenue. In particular, companies no longer have to worry about counterfeit products once effective new drugs are put on the market. Suffer economic losses; consumers can benefit from cost reduction and do not have to worry about buying counterfeit products. It can be seen that the direct and indirect therapeutic and social benefits of the whole society are very huge.
[ad_2]


